Natural gas
Natural gas is a gas consisting primarily of methane. It is found associated with other fossil fuels, in coal beds, as methane clathrates, and is created by methanogenic organisms in marshes, bogs, and landfills. It is an important fuel source, a major feedstock for fertilizers, and a potent greenhouse gas.
Before natural gas can be used as a fuel, it must undergo processing to remove almost all materials other than methane. The by-products of that processing include ethane, propane, butanes, pentanes, and higher molecular weight hydrocarbons, elemental sulfur, carbon dioxide, water vapor, and sometimes helium and nitrogen.
Natural gas is often informally referred to as simply gas, especially when compared to other energy sources such as oil or coal.
Contents |
Sources
Fossil natural gas

In the 1800s, natural gas was usually produced as a byproduct of producing oil, since the small, light gas carbon chains come out of solution as it undergoes pressure reduction from the reservoir to the surface, similar to uncapping a bottle of soda pop where the carbon dioxide effervesces. Unwanted natural gas can be a disposal problem at the well site. If there is not a market for natural gas near the wellhead it was virtually valueless since it must be piped to the end user. In the 1800s and early 1900s, such unwanted gas was usually burned off at the wellsite. Often, unwanted gas (or 'stranded' gas without a market) is pumped back into the reservoir with an 'injection' well for disposal or repressurizing the producing formation. In locations (such as the United States) with a high natural gas demand, pipelines are constructed to take the gas from the wellsite to the end consumer.
Another solution is to export the natural gas as a liquid.[1] Gas-to-liquid, (GTL) is a developing technology that converts stranded natural gas into synthetic gasoline, diesel or jet fuel through the Fischer-Tropsch process developed in World War II Germany. Such fuels can be transported through conventional pipelines and tankers to users. Proponents claim GTL fuels burn cleaner than comparable petroleum fuels. Most major international oil companies are in advanced development stages of GTL production, with a world-scale (140,000 barrels a day) GTL plant in Qatar scheduled to come online before 2010.
Fossil natural gas can be "associated" (found in oil fields) or "non-associated" (isolated in natural gas fields), and is also found in coal beds (as coalbed methane). It sometimes contains significant quantities of ethane, propane, butane, and pentane—heavier hydrocarbons removed prior to use as a consumer fuel—as well as carbon dioxide, nitrogen, helium and hydrogen sulfide.[2]
Natural gas is commercially produced from oil fields and natural gas fields. Gas produced from oil wells is called casinghead gas or associated gas. The natural gas industry is producing gas from increasingly more challenging resource types: sour gas, tight gas, shale gas and coalbed methane.
The world's largest proven gas reserves are located in Russia, with 4.757 × 1013 m³ (1.6 × 1015 cubic feet). Russia is also the world's largest natural gas producer, through the Gazprom company. Major proven resources (with year of estimate) (in billion cubic metres) are world 175,400 (2006), Russia 47,570 (2006), Iran 26,370 (2006), Qatar 25,790 (2007), Saudi Arabia 6,568 (2006) and United Arab Emirates 5,823 (2006). It is estimated that there are also about 900 tetrillion cubic metres of "unconventional" gas such as shale gas, of which 180 tetrillion may be recoverable.[3]
The world's largest gas field is Qatar's offshore North Field, estimated to have 25 trillion cubic metres[4] (9.0 × 1014cubic feet) of gas in place—enough to last more than 200 years at optimum production levels. The second largest natural gas field is the South Pars Gas Field in Iranian waters in the Persian Gulf. Connected to Qatar's North Field, it has estimated reserves of 8 to 14 trillion cubic metres[5] (2.8 × 1014 to 5.0 × 1014 cubic feet) of gas.
Because natural gas is not a pure product, as the reservoir pressure drops when non-associated gas is extracted from a field under supercritical (pressure/temperature) conditions, the higher molecular weight components may partially condense upon isothermic depressurizing—an effect called retrograde condensation. The liquids thus formed may get trapped by depositing in the pores of the gas reservoir. One method to deal with this problem is to reinject dried gas free of condensate to maintain the underground pressure and to allow reevaporation and extraction of condensates.
Town gas
Town gas is a mixture of methane and other gases, mainly the highly toxic carbon monoxide, that can be used in a similar way to natural gas and can be produced by treating coal chemically. This is a historic technology, still used as 'best solution' in some local circumstances, although coal gasification is not usually economic at current gas prices. However, depending upon infrastructure considerations, it remains a future possibility.
Most town "gashouses" located in the eastern United States in the late nineteenth and early twentieth centuries were simple by-product coke ovens which heated bituminous coal in air-tight chambers. The gas driven off from the coal was collected and distributed through town-wide networks of pipes to residences and other buildings where it was used for cooking and lighting purposes. (Gas heating did not come into widespread use until the last half of the twentieth century.) The coal tar that collected in the bottoms of the gashouse ovens was often used for roofing and other water-proofing purposes, and also, when mixed with sand and gravel, was used for creating bitumen for the surfacing of local streets.
Biogas
When methane-rich gases are produced by the anaerobic decay of non-fossil organic matter (biomass), these are referred to as biogas (or natural biogas). Sources of biogas include swamps, marshes, and landfills (see landfill gas), as well as sewage sludge and manure[6] by way of anaerobic digesters, in addition to enteric fermentation particularly in cattle.
Methanogenic archaea are responsible for all biological sources of methane, some in symbiotic relationships with other life forms, including termites, ruminants, and cultivated crops. Methane released directly into the atmosphere would be considered a pollutant. However, methane in the atmosphere is oxidized, producing carbon dioxide and water. Methane in the atmosphere has a half life of seven years, meaning that if a tonne of methane were emitted today, 500 kilograms would have broken down to carbon dioxide and water after seven years.
Other sources of methane, the principal component of natural gas, include landfill gas, biogas and methane hydrate. Biogas, and especially landfill gas, are already used in some areas, but their use could be greatly expanded. Landfill gas is a type of biogas, but biogas usually refers to gas produced from organic material that has not been mixed with other waste.
Landfill gas is created from the decomposition of waste in landfills. If the gas is not removed, the pressure may get so high that it works its way to the surface, causing damage to the landfill structure, unpleasant odor, vegetation die-off and an explosion hazard. The gas can be vented to the atmosphere, flared or burned to produce electricity or heat. Experimental systems were being proposed for use in parts Hertfordshire, UK and Lyon in France.
Once water vapor is removed, about half of landfill gas is methane. Almost all of the rest is carbon dioxide, but there are also small amounts of nitrogen, oxygen and hydrogen. There are usually trace amounts of hydrogen sulfide and siloxanes, but their concentration varies widely. Landfill gas cannot be distributed through utility natural gas pipelines unless it is cleaned up to less than 3% CO2, and a few parts per million H2S, because CO2 and H2S corrode the pipelines.[7] It is usually more economical to combust the gas on site or within a short distance of the landfill using a dedicated pipeline. Water vapor is often removed, even if the gas is combusted on site. If low temperatures condense water out of the gas, siloxanes can be lowered as well because they tend to condense out with the water vapor. Other non-methane components may also be removed in order to meet emission standards, to prevent fouling of the equipment or for environmental considerations. Co-firing landfill gas with natural gas improves combustion, which lowers emissions.
Gas generated in sewage treatment plants is commonly used to generate electricity. For example, the Hyperion sewage plant in Los Angeles burns 8 million cubic feet of gas per day to generate power [8] New York City utilizes gas to run equipment in the sewage plants, to generate electricity, and in boilers.[9] Using sewage gas to make electricity is not limited to large cities. The city of Bakersfield, California uses cogeneration at its sewer plants.[10] California has 242 sewage wastewater treatment plants, 74 of which have installed anaerobic digesters. The total biopower generation from the 74 plants is about 66 MW.[11]
Biogas is usually produced using agricultural waste materials, such as otherwise unusable parts of plants and manure. Biogas can also be produced by separating organic materials from waste that otherwise goes to landfills. Such method is more efficient than just capturing the landfill gas it produces. Using materials that would otherwise generate no income, or even cost money to get rid of, improves the profitability and energy balance of biogas production.
Anaerobic lagoons produce biogas from manure, while biogas reactors can be used for manure or plant parts. Like landfill gas, biogas is mostly methane and carbon dioxide, with small amounts of nitrogen, oxygen and hydrogen. However, with the exception of pesticides, there are usually lower levels of contaminants.
Crystallized natural gas - Hydrates
Huge quantities of natural gas (primarily methane) exist in the form of hydrates under sediment on offshore continental shelves and on land in arctic regions that experience permafrost such as those in Siberia (hydrates require a combination of high pressure and low temperature to form). However, as of 2010[update] no technology has been developed to produce natural gas economically from hydrates.
It costs anywhere between once and twice as much to produce usable natural gas from crystallized natural gas, using current technology.[13]
Natural gas processing
The image below is a schematic block flow diagram of a typical natural gas processing plant. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets.
The block flow diagram also shows how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) propane, butanes and natural gasoline (denoted as pentanes +).[14][15][16][17][18]

Uses
Power generation
Natural gas is a major source of electricity generation through the use of gas turbines and steam turbines. Most grid peaking power plants and some off-grid engine-generators use natural gas. Particularly high efficiencies can be achieved through combining gas turbines with a steam turbine in combined cycle mode. Natural gas burns more cleanly than other fossil fuels, such as oil and coal, and produces less carbon dioxide per unit energy released. For an equivalent amount of heat, burning natural gas produces about 30% less carbon dioxide than burning petroleum and about 45% less than burning coal.[19] Combined cycle power generation using natural gas is thus the cleanest source of power available using fossil fuels, and this technology is widely used wherever gas can be obtained at a reasonable cost. Fuel cell technology may eventually provide cleaner options for converting natural gas into electricity, but as yet it is not price-competitive.
Domestic use
Natural gas is supplied to homes where it is used for such purposes as cooking in natural gas-powered ranges and ovens, natural gas-heated clothes dryers, heating/cooling and central heating. Home or other building heating may include boilers, furnaces, and water heaters. CNG is used in rural homes without connections to piped-in public utility services, or with portable grills. Natural gas is also supplied by independent natural gas suppliers through Natural Gas Choice programs throughout the United States. However, due to CNG being less economical than LPG, LPG (propane) is the dominant source of rural gas.

Transportation
Compressed natural gas (methane) is a cleaner alternative to other automobile fuels such as gasoline (petrol) and diesel. As of 2008 there were 9.6 million natural gas vehicles worldwide, led by Pakistan (2.0 million), Argentina (1.7 million), Brazil (1.6 million), Iran (1.0 million), and India (650,000).[20][21] The energy efficiency is generally equal to that of gasoline engines, but lower compared with modern diesel engines. Gasoline/petrol vehicles converted to run on natural gas suffer because of the low compression ratio of their engines, resulting in a cropping of delivered power while running on natural gas (10%-15%). CNG-specific engines, however, use a higher compression ratio due to this fuel's higher octane number of 120–130.[22]
Fertilizers
Natural gas is a major feedstock for the production of ammonia, via the Haber process, for use in fertilizer production.
Aviation
Russian aircraft manufacturer Tupolev is currently running a development program to produce LNG- and hydrogen-powered aircraft.[23] The program has been running since the mid-1970s, and seeks to develop LNG and hydrogen variants of the Tu-204 and Tu-334 passenger aircraft, and also the Tu-330 cargo aircraft. It claims that at current market prices, an LNG-powered aircraft would cost 5,000 roubles (~ $218/ £112) less to operate per ton, roughly equivalent to 60%, with considerable reductions to carbon monoxide, hydrocarbon and nitrogen oxide emissions.
The advantages of liquid methane as a jet engine fuel are that it has more specific energy than the standard kerosene mixes do and that its low temperature can help cool the air which the engine compresses for greater volumetric efficiency, in effect replacing an intercooler. Alternatively, it can be used to lower the temperature of the exhaust.
Hydrogen
Natural gas can be used to produce hydrogen, with one common method being the hydrogen reformer. Hydrogen has various applications: it is a primary feedstock for the chemical industry, a hydrogenating agent, an important commodity for oil refineries, and a fuel source in hydrogen vehicles.
Other
Natural gas is also used in the manufacture of fabrics, glass, steel, plastics, paint, and other products.
Storage and transport

Because of its low density, it is not easy to transport or store natural gas. Natural gas pipelines are impractical across oceans. Many existing pipelines in North America are close to reaching their capacity, prompting some politicians representing northern states to speak of potential shortages. In Europe, the gas pipeline network is already dense in the West[24]. New pipelines are planned or under construction in Eastern Europe and between gas fields in Russia, Near East and Northern Africa and Western Europe. See also List of natural gas pipelines.
LNG carriers transport liquefied natural gas (LNG) across oceans, while tank trucks can carry liquefied or compressed natural gas (CNG) over shorter distances. Sea transport using CNG carrier ships that are now under development may be competitive with LNG transport in specific conditions.
Gas is turned into liquid at a liquefaction plant, and is returned to gas form at regasification plant at the terminal. Shipborne regasification equipment is also used. LNG is the preferred form for long distance, high volume transportation of natural gas, whereas pipeline is preferred for transport for distances up to 4,000 km over land and approximately half that distance offshore.
CNG is transported at high pressure, typically above 200 bars. Compressors and decompression equipment are less capital intensive and may be economical in smaller unit sizes than liquefaction/regasification plants. Natural gas trucks and carriers may transport natural gas directly to end-users, or to distribution points such as pipelines.

In the past, the natural gas which was recovered in the course of recovering petroleum could not be profitably sold, and was simply burned at the oil field in a process known as flaring. Flaring is now illegal in many countries.[25] Additionally, companies now recognize that gas may be sold to consumers in the form of LNG or CNG, or through other transportation methods. The gas is now re-injected into the formation for later recovery. The re-injection also assists oil pumping by keeping underground pressures higher.
A "master gas system" was invented in Saudi Arabia in the late 1970s, ending any necessity for flaring. Satellite observation, however, shows that flaring[26] and venting[27] are still practiced in some gas-producing countries.
Natural gas is used to generate electricity and heat for desalination. Similarly, some landfills that also discharge methane gases have been set up to capture the methane and generate electricity.
Natural gas is often stored underground inside depleted gas reservoirs from previous gas wells, salt domes, or in tanks as liquefied natural gas. The gas is injected in a time of low demand and extracted when demand picks up. Storage nearby end users helps to meet volatile demands, but such storage may not always be practicable.
With 15 countries accounting for 84% of the worldwide production, access to natural gas has become an important issue in international politics, and countries vie for control of pipelines.[28] In the 2000s, Gazprom, the state-owned energy company in Russia, engaged in disputes with Ukraine and Belarus over the price of natural gas, which have created worries that gas deliveries to parts of Europe could be cut off for political reasons.[29]
Environmental effects
CO2 emissions
Natural gas is often described as the cleanest fossil fuel, producing less carbon dioxide per joule delivered than either coal or oil.[19], and far fewer pollutants than other fossil fuels. However, in absolute terms, it does contribute substantially to global carbon emissions, and this contribution is projected to grow. According to the IPCC Fourth Assessment Report (Working Group III Report, chapter 4), in 2004, natural gas produced about 5.3 billion tons a year of CO2 emissions, while coal and oil produced 10.6 and 10.2 billion tons respectively (figure 4.4). According to an updated version of the SRES B2 emissions scenario, however, by the year 2030, natural gas would be the source of 11 billion tons a year, with coal and oil now 8.4 and 17.2 billion respectively because demand is increasing 1.9% a year[30] (Total global emissions for 2004 were estimated at over 27,200 million tons).
In addition, natural gas itself is a greenhouse gas more potent than carbon dioxide when released into the atmosphere, although natural gas is released in much smaller quantities. However, Natural Gas has an effect in the ozone for approximately 12 years, while C02 has an effect for 100 to 500 years. Natural gas is mainly composed of methane, which has a radiative forcing twenty times greater than carbon dioxide. Based on such composition, a ton of methane in the atmosphere traps in as much radiation as 20 tons of carbon dioxide. Carbon dioxide still receives the lion's share of attention over greenhouse gases because it is released in much larger amounts. Still, it is inevitable when natural gas is used on a large scale that some of it will leak into the atmosphere. Current estimates by the EPA place global emissions of methane at 3 trillion cubic feet annually,[31] or 3.2% of global production.[32] Direct emissions of methane represented 14.3% of all global anthropogenic greenhouse gas emissions in 2004.[33]
Other pollutants
Natural gas produces far lower amounts of sulfur dioxide and nitrous oxides than any other fossil fuel. Carbon Dioxide produced is 117,000 ppm vs 208,000 for burning coal. Carbon Monoxide produced is 40 ppm vs 208 for burning coal. Nitrogen Oxides produced is 92 ppm vs 457 for burning coal. Sulfer Dioxide is 1 ppm vs 2,591 for burning coal. Particulates are 7 ppm vs 2,744 for burning coal. Mercury is 0 vs .016 for burning coal. http://www.global-greenhouse-warming.com/gas-vs-coal.html Particulates are also a major contribution to global warming. Natural gas has 7ppm vs coals 2,744ppm ((http://www.physicalgeography.net/fundamentals/7h.html))
Safety
In any form, a minute amount of odorant such as t-butyl mercaptan, with a rotting-cabbage-like smell, is added to the otherwise colorless and almost odorless gas, so that leaks can be detected before a fire or explosion occurs. Sometimes a related compound, thiophane is used, with a rotten-egg smell. Adding odorant to natural gas began in the United States after the 1937 New London School explosion. The buildup of gas in the school went unnoticed, killing three hundred students and faculty when it ignited. Odorants are considered non-toxic in the extremely low concentrations occurring in natural gas delivered to the end user.
In mines, where methane seeping from rock formations has no odor, sensors are used, and mining apparatuses have been specifically developed to avoid ignition sources such as the Davy lamp.
Explosions caused by natural gas leaks occur a few times each year. Individual homes, small businesses and boats are most frequently affected when an internal leak builds up gas inside the structure. Frequently, the blast will be enough to significantly damage a building but leave it standing. In these cases, the people inside tend to have minor to moderate injuries. Occasionally, the gas can collect in high enough quantities to cause a deadly explosion, disintegrating one or more buildings in the process. The gas usually dissipates readily outdoors, but can sometimes collect in dangerous quantities if weather conditions are right. However, considering the tens of millions of structures that use the fuel, the individual risk of using natural gas is very low.
Some gas fields yield sour gas containing hydrogen sulfide (H2S). This untreated gas is toxic. Amine gas treating, an industrial scale process which removes acidic gaseous components, is often used to remove hydrogen sulfide from natural gas.[34]
Extraction of natural gas (or oil) leads to decrease in pressure in the reservoir. Such decrease in pressure in turn may result in subsidence at ground level. Subsidence may affect ecosystems, waterways, sewer and water supply systems, foundations, and so on.
Natural gas heating systems are a minor source of carbon monoxide deaths in the United States. According to the US Consumer Product Safety Commission (2008), 56% of unintentional deaths from non-fire CO poisoning were associated with engine-driven tools like gas-powered generators and lawn mowers. Natural gas heating systems accounted for 4% of these deaths. Improvements in natural gas furnace designs have greatly reduced CO poisoning concerns. Detectors are also available that warn of carbon monoxide and/or explosive gas (methane, propane, etc.).
Energy content, statistics and pricing
Quantities of natural gas are measured in normal cubic meters (corresponding to 0°C at 101.325 kPa) or in standard cubic feet (corresponding to 60 °F (16 °C) and 14.73 psia). The gross heat of combustion of one cubic meter of commercial quality natural gas is around 39 megajoules (≈10.8 kWh), but this can vary by several percent. This comes to about 49 megajoules (≈13.5 kWh) for one kg of natural gas (assuming 0.8 kg/m^3, an approximate value).
The price of natural gas varies greatly depending on location and type of consumer. In 2007, a price of $7 per 1,000 cubic feet (28 m3) was typical in the United States. The typical caloric value of natural gas is roughly 1,000 British thermal units (BTU) per cubic foot, depending on gas composition. This corresponds to around $7 per million BTU, or around $7 per gigajoule. In April 2008, the wholesale price was $10 per 1,000 cubic feet (28 m3) ($10/MMBTU).[35] The residential price varies from 50% to 300% more than the wholesale price. At the end of 2007, this was $12–$16 per 1,000 cu ft (28 m3).[36] Natural gas in the United States is traded as a futures contract on the New York Mercantile Exchange. Each contract is for 10,000 MMBTU (~10,550 gigajoules), or 10 billion BTU. Thus, if the price of gas is $10 per million BTUs on the NYMEX, the contract is worth $100,000.
United Kingdom
Natural gas is also traded as a commodity in Europe, principally at the United Kingdom NBP and related European hubs, such as the TTF in the Netherlands.
European Union
As one of the world's largest importers of natural gas, the EU is a major player on the international gas market. With Norway being one of the world's largest suppliers of natural gas as part of the extended European Economic Area, most discussions can be conducted within the EU. The main supplier is then expected to be the current number two: the Russian Federation.
Gas prices for end users vary greatly across the EU.[37]. A single European energy market, one of the key objectives of the European Union, should level the prices of gas in all EU member states.
United States
In US units, one standard cubic foot of natural gas produces around 1,028 British thermal units (BTU). The actual heating value when the water formed does not condense is the net heat of combustion and can be as much as 10% less.[38]
In the United States, retail sales are often in units of therms (th); 1 therm = 100,000 BTU. Gas meters measure the volume of gas used, and this is converted to therms by multiplying the volume by the energy content of the gas used during that period, which varies slightly over time. Wholesale transactions are generally done in decatherms (Dth), or in thousand decatherms (MDth), or in million decatherms (MMDth). A million decatherms is roughly a billion cubic feet of natural gas.
As of 2009, the Potential Gas Committee estimated that the United States has total future recoverable natural gas resources approximately 100 times greater than current annual consumption.[39]
Elsewhere
In the rest of the world, LNG (liquified natural gas) and LPG (liquified petroleum gas) is traded in metric tons or mmBTU as spot deliveries. Long term contracts are signed in metric tons. The LNG and LPG is transported by specialized transport ships, as the gas is liquified at cryogenic temperatures. The specification of each LNG/LPG cargo will usually contain the energy content, but this information is in general not available to the public.
See also
|
|
|
References
- ↑ Gas to liquid
- ↑ Natural gas overview
- ↑ "Wonderfuel: Welcome to the age of unconventional gas" by Helen Knight, New Scientist, 12 June 2010, pp. 44-7.
- ↑ Background note: Qatar
- ↑ "Pars Special Economic Energy Zone". Pars Special Economic Energy Zone. http://www.pseez.ir/gas-en.html. Retrieved 2007-07-17.
- ↑ http://www.manure.umn.edu/
- ↑ http://www.beg.utexas.edu/energyecon/lng/documents/CEE_Interstate_Natural_Gas_Quality_Specifications_and_Interchangeability.pdf
- ↑ http://www.lasewers.org/treatment_plants/hyperion/index.htm
- ↑ http://www.nyc.gov/html/dep/pdf/wwsystem.pdf
- ↑ http://www.parsons.com/projects/Pages/bakersfield-wwtp-3.aspx
- ↑ http://www.energy.ca.gov/2010publications/CEC-500-2010-007/CEC-500-2010-007.PDF
- ↑ http://www.naturalgas.org/images/McMahon-Plnt.jpg on http://www.naturalgas.org/naturalgas/processing_ng.asp
- ↑ Fortune Magazine - Frozen Natural Gas in Indian Ocean
- ↑ Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market
- ↑ Example Gas Plant
- ↑ From Purification to Liquefaction Gas Processing
- ↑ Feed-Gas Treatment Design for the Pearl GTL Project
- ↑ Benefits of integrating NGL extraction and LNG liquefaction
- ↑ 19.0 19.1 Natural Gas and the Environment
- ↑ "Natural Gas Vehicle Statistics". International Association for Natural Gas Vehicles. http://www.iangv.org/tools-resources/statistics.html. Retrieved 2009-10-19.
- ↑ Pike Research (2009-10-19). "Forecast: 17M Natural Gas Vehicles Worldwide by 2015". Green Car Congress. http://www.greencarcongress.com/2009/10/forecast-17m-natural-gas-vehicles-worldwide-by-2015.html#more. Retrieved 2009-10-19.
- ↑ Clean Engine Vehicle, Measurement and Control Laboratory
- ↑ PSC Tupolev - Development of Cryogenic Fuel Aircraft
- ↑ Gas Infrasturcture Europe, retrieved June 18. 2009
- ↑ Hyne, Norman J. (1991). Dictionary of petroleum exploration, drilling & production. pg. 190: PennWell Books. pp. 625. ISBN 0878143521.
- ↑ Satellite observation of flares in the world
- ↑ satellite observation of methane in earth's atmosphere
- ↑ The Contours of the New Cold War
- ↑ Gazprom and Russian Foreign Policy
- ↑ [1]
- ↑ nytimes.com: Curbing Emissions by Sealing Gas Leaks
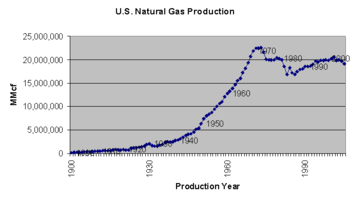
- ↑ Wolfram Alpha query: "World Natural Gas Production"
- ↑ US EPA: Climate Economics
- ↑ NaturalGas.org - Processing Natural Gas
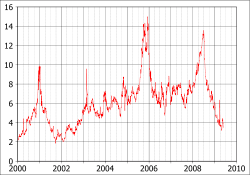
- ↑ Graph of Natural Gas Futures Prices - NYMEX
- ↑ Natural Gas Prices published by the US government
- ↑ EU Gas Prices
- ↑ Heat value definitions. WSU website. Retrieved 2008-05-19.
- ↑ Potential Gas Committee, Potential Gas Committee reports unprecedented increase in magnitude of U.S. natural gas resource base, 18 Jume 2009, *.PDF file.
External links
- CERA - collection of market and industry reports
- DOE/EIA EIA Natural Gas Weekly Update - current NG prices and market analysis
- Natural Gas Media- Natural Gas News and Analysis for Investment and Trading
